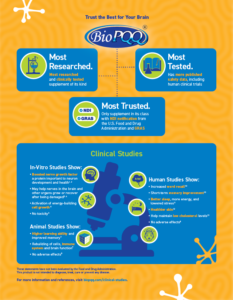
When choosing a nutritional supplement, it’s critical to ensure that ingredients meet food grade standards, are proven safe and can be legally marketed. BioPQQ® is naturally derived from fermentation using proprietary technology that has been well studied and documented. In fact, BioPQQ is the only supplement of its kind with FDA’s NDI notification and GRAS designation. It has been fully characterized and widely tested in animals and humans to support its efficacy and safety.
See the infographics below for more information.


Download Brain Health Infographic
Download Clinical Studies Infographic
Download Energy Infographic
Download Memory Infographic
References
MGCPQQ Reports


Download the BioPQQ Mitochondria Bulletin
History
1979: The Pyrroloquinoline Quinone (PQQ) compound is discovered as coenzyme of oxidoreductase.
1980s: Mitsubishi Gas Chemical begins supplying PQQ disodium salt.
2003: A research group in Japan suggests that the ingredient has the potential to become a new vitamin.
2008: BioPQQ® is successfully filed as a New Dietary Ingredient (NDI) with the U.S. Food & Drug Administration (FDA), and Mitsubishi Gas Chemical becomes the first company to utilize PQQ in food applications. To this day, BioPQQ® remains the only ingredient of its kind with NDI notification.
2014: BioPQQ® is certified as food ingredient for food application by Japan’s MHLW (Ministry of Health, Labour and Welfare).
2017: The EFSA (European Food Safety Authority) completes a safety evaluation on MGCPQQ®, the European sister brand of BioPQQ®, and the evaluation report is made public.
2018: BioPQQ® is certified by the Informed-Choice and Informed-Sport quality assurance programs, ensuring that the ingredient is manufactured to high-quality standards and that every batch is tested for substances banned from the World Anti-Doping Agency (WADA) list. The certifications attest to the ingredient’s quality and manufacturing integrity and reassure athletes that BioPQQ® is safe to use.
2018: MGCPQQ® (BioPQQ®) is listed on the EU’s Approved List of Novel Foods as PQQ, and the ingredient is approved as a food supplement. The Novel Foods approval is exclusive to MGCPQQ®.
Present: BioPQQ® is widely used as a food supplement for its various health maintenance functionalities in U.S.A. and Japan, and the ingredient is made available for sale in Europe for the first time.






